Pigmentation in freshwater isopods: visual predation vs. developmental plasticity
Divergent selection and adaptive phenotypic plasticity can jointly influence phenotypic differentiation within and among populations. Understanding how these processes interact is difficult when the same environmental differences that lead to divergent selection can also promote phenotypic plasticity. Previous work has shown that pigmentation of the freshwater isopod Asellus aquaticus varies with background colour (Hargeby et al. 2004). This is hypothesized to be an evolutionary response to visual predation along a gradient of differently coloured macrophyte-backgrounds.

In an six month long outdoor mesocosm experiment, we investigated how predator density (0, 30, and 60 threespine stickleback per mesocosm) and macrophytes (presence/absence) affected the abundance and pigmentation of isopods. While we found that fish presence strongly reduced isopod density, particularly in the absence of macrophytes, we found no effect on the size or pigmentation of isopods (Lürig et al. 2019). Instead, we detected increased pigmentation in the presence of macrophytes, and across all levels of fish density. A subsequent laboratory rearing experiment indicated that pigmentation of isopods may a developmentally plastic trait.
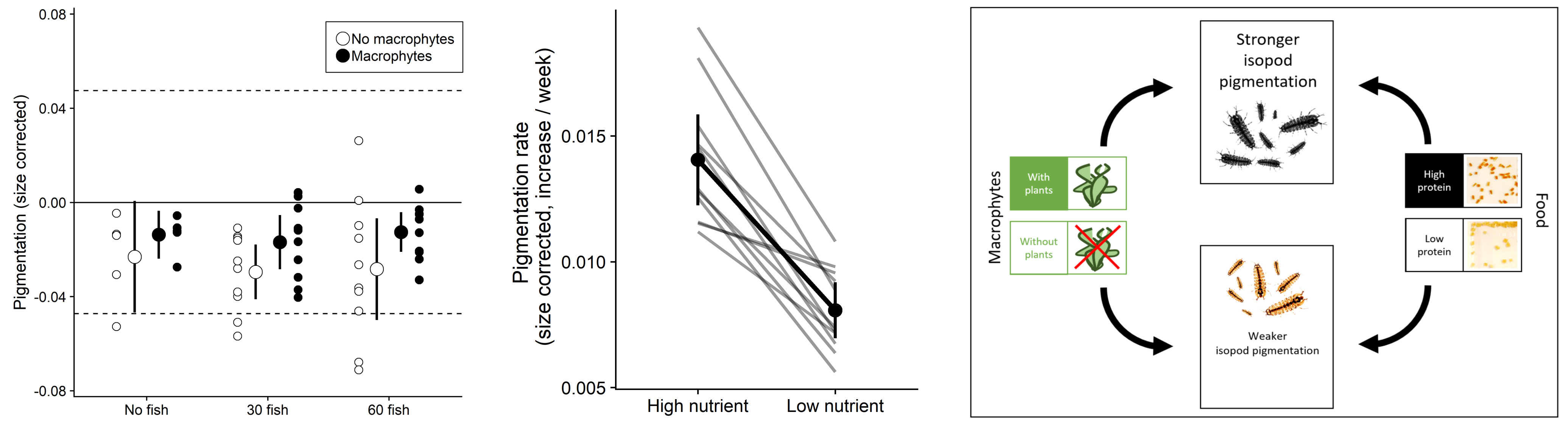
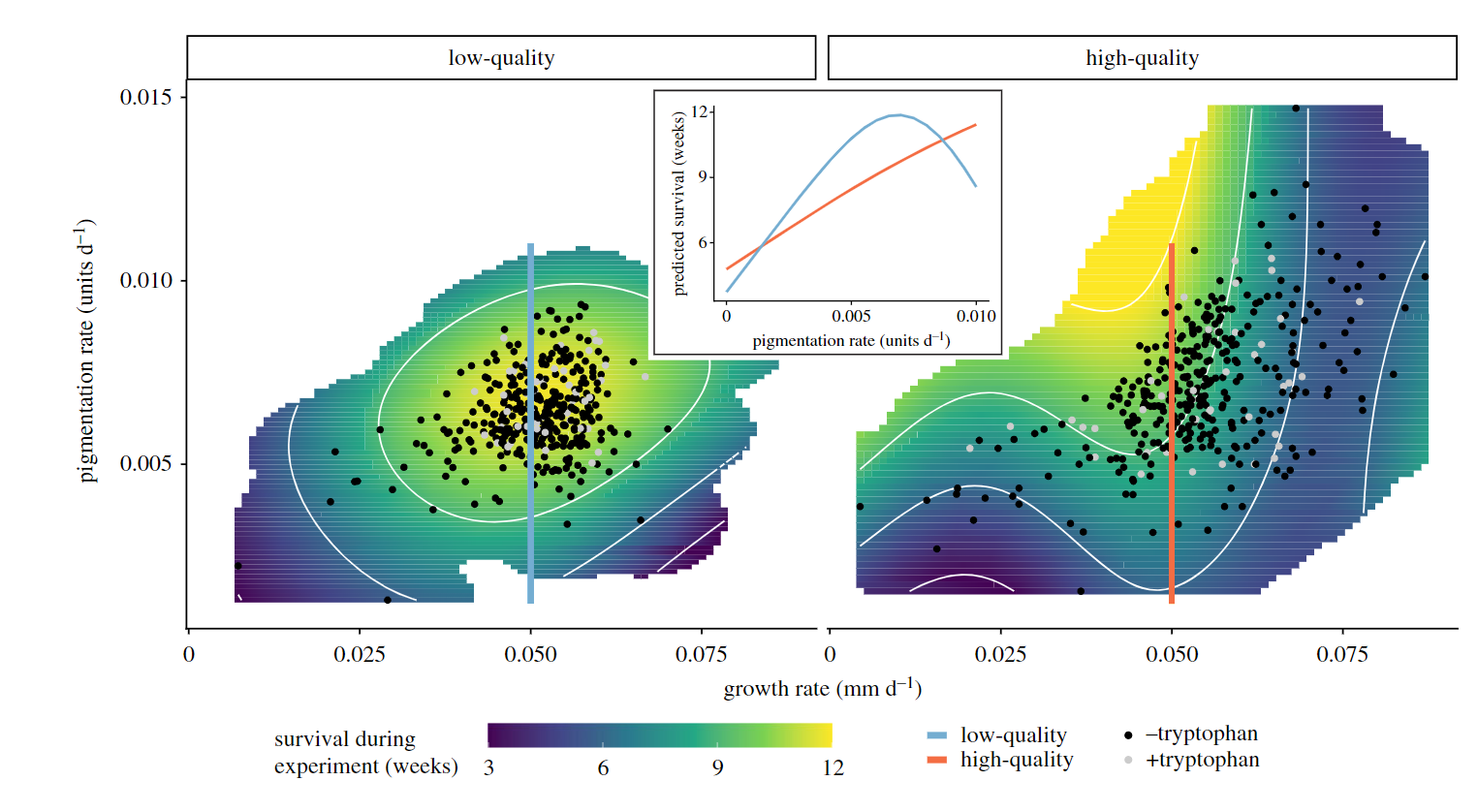
Inspired by the findings of our mesocom experiment and the associated laboratory study, we further investigated how diet driven developmental phenotypic plasticity could be result in divergent patterns of pigmentation. In a large scale laboratory experiment we reared over 1000 individuals from 29 families with two diets of contrasting nutritional quality (low/high protein concentration), and quantified developmental trajectories of body size and pigmentation for every individual for 12 weeks.

Overall, dietary protein had strong effects on the developing phenotype, and this influenced the “survival landscape” of juvenile isopods (Lürig and Matthews 2021). Specifically, we found that higher dietary protein had strong positive effects on the developmental rate of pigmentation, but led to increased mortality under high growth and pigmentation rates. Building on previous work, suggesting that visual predation has mediated the evolution of A. aquaticus body size and cryptic pigmentation, our study shows how dietary effects on the developmental trajectories of juvenile isopods can have fitness consequences in the absence of predation.
![]()
In summary, we found that genetic background and environment experienced during early development interactively shape life history, population demography, and phenotypic variation in isopods. However, the work continues as new questions emerge and the isopod-community becomes more connected (visit https://asellus.org).
![]()
Findings from this and other work on the relevance of isopod pigmentation and development are summarized in a review article on A. aquaticus that we published in 2021 (Lafuente et al. 2021).
References
Hargeby, A., J. Johansson, and J. Ahnesjö. (2004). Habitat-specific pigmentation in a freshwater isopod: adaptive evolution over a small spatiotemporal scale. Evolution; international journal of organic evolution 58:81–94.
Lafuente, E., Lürig, M.D., Rövekamp, M., Matthews, B., Buser, C., Vorburger, C., & Räsänen, K. (2021). Building on 150 years of knowledge: the freshwater isopod Asellus aquaticus as an integrative eco-evolutionary model system. Frontiers in Ecology and Evolution, 699.
Lürig, M.D., R. J. Best, M. Svitok, J. Jokela, and B. Matthews (2019). The role of plasticity in the evolution of cryptic pigmentation in a freshwater isopod. The Journal of Animal Ecology 88:612–623.
Lürig, M.D., and Matthews, B. (2021). Dietary-based developmental plasticity affects juvenile survival in an aquatic detritivore. Proceedings of the Royal Society B: Biological Sciences, 288(1945), 20203136.